Natural disasters are becoming more frequent. Weather patterns that would have been an anomaly 50 years ago are now normal. From extremely high temperatures and droughts to floods and flash freezes, the climate seems more unstable than ever. The results? Whole agricultural regions devastated, local economies destroyed, lives lost – and if we don’t act fast to prevent the situation from worsening and to adapt efficiently, our global, interconnected culture might be threatened. In July 2022, the UK underwent its first-ever red extreme weather warming as part of the European Heat Wave, which also caused wildfires and hundreds of deaths across Spain and Portugal.
The key culprit to the aforementioned issues is climate change, caused by an increased concentration of atmospheric greenhouse gases (GHGs) due to human emissions. GHGs are gaseous molecules with the property to absorb heat and emit back into the atmosphere, thus “trapping” the energy we get from the Sun instead of letting it escape. They are released by human activities, mostly the burning of fossil fuels for energy and transportation, but also by various industries, by land clearing, by agriculture and others. Hundreds of gases can be classified as GHGs, but one in particular is responsible for both historical warming anomalies and for present-day changes: carbon dioxide (CO2). If we could reduce CO2 in the atmosphere, we might be able to prevent the climate change.
Splitting concepts
Splitting (sometimes called “cracking”) CO2 refers to the theoretical reaction of breaking a CO2 molecule into pure carbon and oxygen.
Turning CO2 to carbon and oxygen is of high value – the potential benefits range from financial, because pure carbon has many applications, to environmental, due to the potential use of the reaction to reduce CO2 emissions. Highly lucrative applications can be found for the solid carbon generated by the process, such as building high-performance vehicles, making electrodes for batteries, designing advanced construction materials, producing superconductors and making synthetic catalysts. It would allow certain industries to mitigate their emissions while generating valuable materials, thus giving a strong financial incentive to reduce environmental impact. This has resulted in high scientific interest; many approaches are being explored in the process.
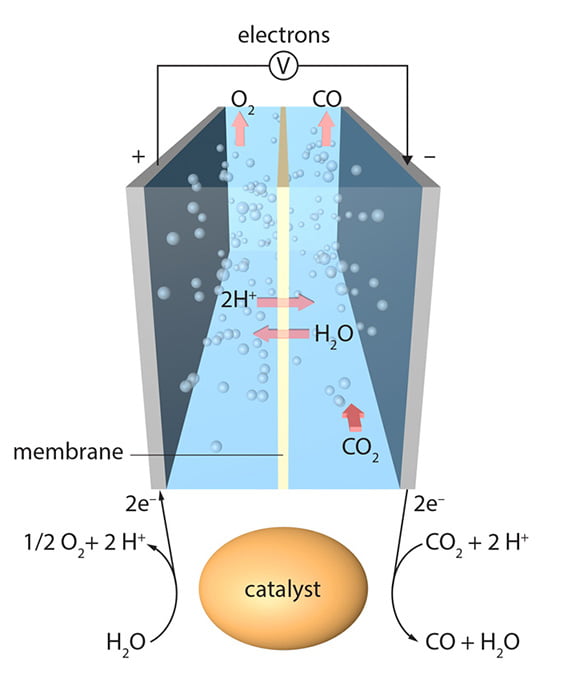
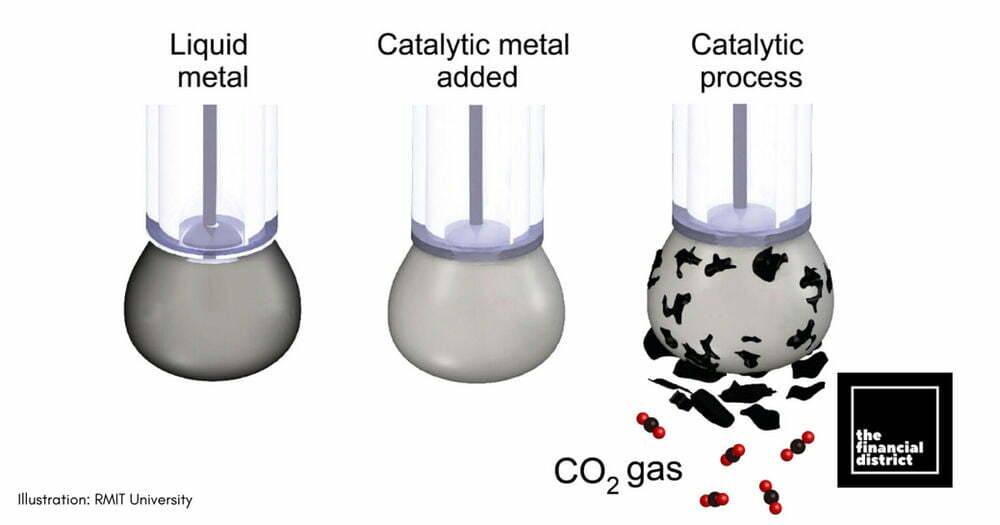
Gallium-based liquid metal catalysts
There are several scientific advancements in this area. Some experiments led by Australian researchers are using Gallium-based liquid metal catalysts. The cost is competitive, and the conversion can be done at room temperature so that it would be feasible to power the reaction with renewable energy. The products are pure oxygen and carbonaceous materials, which contain a mix of pure carbon, oxygen and traces of the metals used to facilitate the reaction. However, the technology is still at laboratory scale, and there are several risks including low yields (milligrams of carbon at the most at this stage), low energy efficiency, complicated processes, the toxicity or ‘low abundance’ of some materials used during the process, and high capital or running costs according to Ali Zavabeti from University of Melbourne. The researchers are finding ways to improve it and the next step is to build a larger system in the hope of commercialization. “There are very high rewards by successful upscaling the process” explains Ali, hinting at potential future wide-scale use.
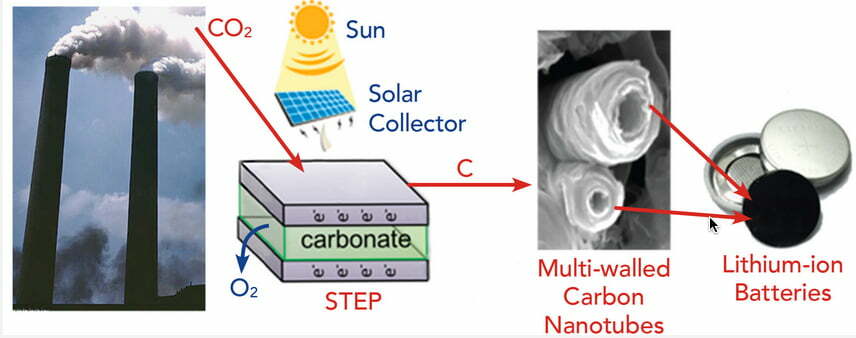
Turning CO2 into carbon nanotubes
Another technique uses lithium carbonate and turn CO2 into carbon nanotubes (CNTs), which are highly valuable and rare. Nonetheless, lithium is rare, expensive and its extraction damages the environment. Wang et. al develop an alternative method using non-lithiated electrolytes, which has a high yield. The process requires close supervision and precision tuning, due to needing several parameters to be met for the reaction to be optimal, so sadly industrial-level use might not be feasible as a way to fight climate change, yet it is still a great way of obtaining CNT.
Converting CO2 into carbon black powder
Another novel method has been developed by the Karlsruhe Institute of Technology (KIT), which has built the world’s first container-scale facility to convert CO2 from the ambient air into pure carbon black powder with the help of renewably sourced hydrogen. The drawback is that reaction is highly complex and contains several steps, and it hasn’t been streamlined in a singular process yet, thus making it difficult to upgrade its scale for the time being.

Turning CO2 into carbon compounds
Instead of generating carbon in the end, a technically easier approach to capture carbon is to turn it into carbon compounds such as methane, CO, and methanol. Some of these compounds are natural fuels and can replace conventional petroleum feedstocks (oil). By using already-existing CO2 as a raw resource, they would avoid the environmental damage of fossil fuels and bypass the issue of limited stocks. Others are sought-after chemicals, needed in various industries. Wide-ranging research has been done in this area with the most visible material results: industrial plants are to be built in the near future based on this technology, with several already in the planning stage.
For example, Evonik and Siemens conducted a joint project called Rheticus to generate specialty chemicals from hydrogen and CO2 based on electrolysis and fermentation processes. The pilot plant has been put into operations and “In about five years’ time (2027), an industrial plant based on this technology is expected to produce 10,000 tons butanol per year using 25,000 tons of CO2 as feedstock”, told us Evgeny Rebrov.
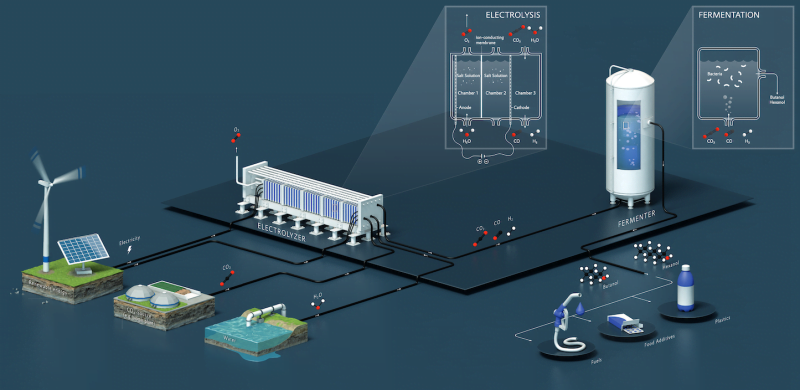
Moreover, a photocatalytic and electrocatalytic CO2-to-fuel production led by Tianyi Ma has gone through lab scale, bench scale, and is ready for micro-pilot plant production, with a ton-level CO2 processing micro-pilot plant being constructed in Victoria.
Problems
However, there are still problems to be solved such as high energy required, low yield and instability of catalysts, splitting with H2 intrinsically low efficiency due to the chemical and physical properties of the materials, etc.
While climate change remains a very grave predicament that will require a variety of approaches for successful mitigation and adaption, including radical transformations in our culture, technological developments and economical restructuring, all focused on reducing emissions, carbon splitting remains a potentially promising way to dispose of the excess CO2 emissions that cannot be avoided. The technologies discussed, while under development, are all helping us bridge the knowledge gap towards efficient, commercially viable and reliable carbon splitting. Successfully developing industrial carbon splitting will no doubt play a key role in a holistic approach to climate change mitigation.
Information in the text is used from several sources (BBC, the Journal Energy & Environmental Science, Research Gate, Energy Live News, Advanced Science News, Ali Zavabeti, Evgeny Rebrov, and Tianyi Ma)
This article was created as part of the TeaMWork program that brings together students across the globe (Monash University in Australia and Malaysia, and the University of Warwick in the United Kingdom).
Authors: Andrei-Alexandru Mihail and Kehan Yan, University of Warwick, UK


