T cells are immune system warriors that circulate in blood to fight infections. CAR T-cell therapy is an inventive treatment that takes T cells from the patient’s body and modifies them to destroy cancer cells. CAR T-cell therapy, a type of immunotherapy, is a particular breakthrough for children with leukemia, and for adults with certain hard-to-treat blood cancers. It also holds promise for other diseases.
Doctors at the OHSU Knight Cancer Institute have been at the forefront of clinical trials of this therapy. We’re building an expert team to make this complex treatment available to patients across the region.
- OHSU was the first hospital in the Northwest to offer patients Kymriah, the first CAR T-cell therapy approved by the Food and Drug Administration.
- A Knight Cancer Institute doctor co-led the first study to outline Kymriah’s long-term effectiveness.
- Our doctors helped lead the clinical trial showing that CAR T-cell therapy for lymphoma not only extends survival but may improve quality of life after treatment.
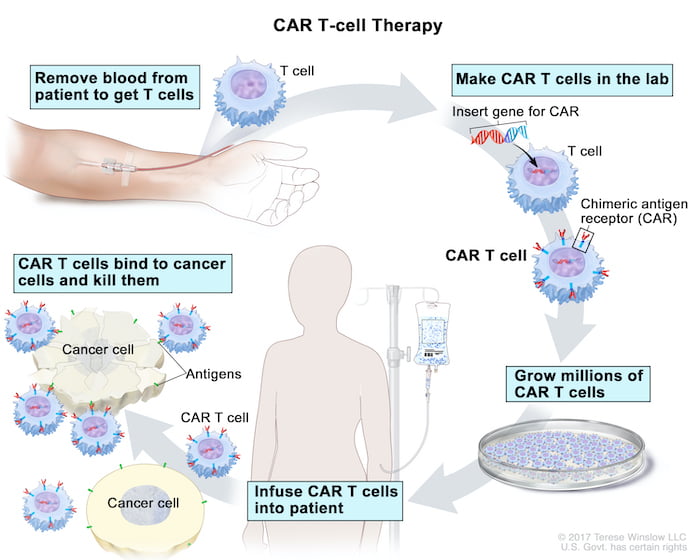
What is CAR T-cell therapy?
CAR T-cell therapy is a complex, multistep treatment. It’s best handled at centers — such as the Knight Cancer Institute — with deep experience doing stem cell and bone marrow transplants (they have done more than 4,500 transplants), so let’s start with some definitions.
- T cells are lymphocytes — a type of white blood cell that fights infection as part of the immune system.
- CAR (chimeric antigen receptor) is a new kind of protein. “Receptor” means it can bind to a cancer cell, like a key fitting in a lock.
- CAR T-cell therapy is a gene therapy because genes in the patient’s T cells are reprogrammed to make CARs.
How CAR T-cell therapy works?
How T cells are collected: Treatment starts with collecting T cells from the patient’s blood. Blood is drawn through one IV line and filtered to collect T cells. The rest is returned through another IV line.
How T cells are modified: The T cells are sent to a lab where they are genetically modified to make the chimeric antigen receptor, or CAR. This protein binds to specific proteins on the surface of cancer cells. That equips the modified T cells to target and kill the cancer cells.
Treatment: The lab grows millions of the modified T cells over several weeks, and ships them back to the hospital. Doctors may give the patient a short course of chemotherapy before giving them the CAR T cells in an IV drip. Once in the patient’s bloodstream, the CAR T cells multiply and may be able to destroy all the cancer cells.
Results
The therapy has led to long-term remission for children and adults with some types of leukemia and lymphoma. The reprogrammed cells may stay in the body to fight cancer for years. CAR T-cell therapy success rate is about 30% to 40% for lasting remission, with no additional treatment.
Side effects
Some people have had serious side effects, including high fevers and dangerously low blood pressure. Other serious side effects include brain swelling, confusion, seizures and severe headaches. Experienced centers such as the Knight Cancer Institute have learned how to recognize and treat side effects early.
Research
OHSU researchers are studying using donor T cells — instead of the patient’s own cells — for CAR T-cell therapy. Donor cells may be more effective at fighting cancer. We are also researching how to use this therapy for other diseases, such as sarcoma.


