Human disease networks and disease gene networks are used to organize a tremendous amount of medical knowledge. But can these tools also give us new clues regarding cures and treatments?
Scientists have spent decades mapping human disease genes. Initially, most of this effort was focused on the identification of genetic mutations responsible for single-gene disorders, like Duchenne muscular dystrophy. More recently, however, new technology has made it possible to link multiple genes to a single disease, as well as to connect multiple diseases to one another by way of the genes associated with them. Genome-wide association studies (GWAS) have played a large role in unraveling these complex relationships. Although these studies do not account for the many environmental factors that contribute to disease, they have revealed numerous gene-disease associations. In addition, such efforts have encouraged health care professionals to think about the molecular pathways of disease, which in turn has led to the development of various new treatment options.
Of course, researchers still have a long way to go before much of the knowledge provided by GWAS can actually be used to treat or cure human disorders. One prominent obstacle along the path to this goal involves determining how to best manage the ever-growing body of gene association data.
Organizing Gene Association Data
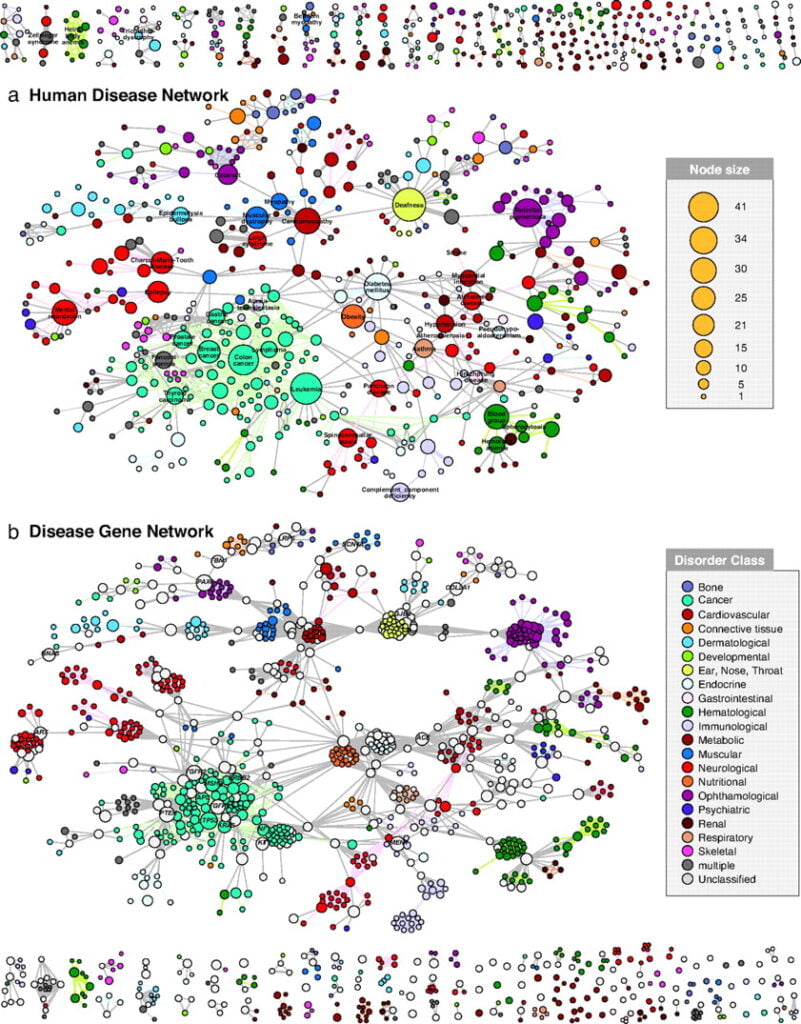
As an initial effort toward organization of the vast and continually expanding set of data obtained through GWAS, a group of scientists from several research institutions in the United States and Korea have developed what they call the “human disease network,” a visual map of all human diseases with known underlying genetic associations; this map also details the genetic connections between these diseases. The map is made up of nodes and branches. Each node represents a disease, and the size of the node reflects the number of genes known to be associated with that disorder. The thickness of the branches that connect various nodes is a reflection of the number of genes shared by the connected diseases.
The idea behind the map is that most diseases with known genetic associations seem to share most of their genes with other diseases. This is often true of diseases that you would probably never imagine have anything to do with each other–such as type 2 diabetes and prostate cancer. Believe it or not, both of these conditions appear to be influenced by variation in the JAZF1 gene (Zeggini et al., 2008). As Dr. Francis S. Collins, Director of the National Human Genome Research Institute, was quoted in a New York Times article, “I’m shaking my head with disbelief that two genes would pop up in these two diseases that have absolutely nothing in common” (Pollack, 2008).
By mapping the genetic connections among diseases, scientists hope they will be able to learn more about the molecular pathways that cause disease–not just to satisfy their own curiosity, but also to aid in the design and development of new ways of treating or curing disease. But how? Quite simply, biologists hope that by knowing when two diseases are connected through genetic associations, they might find instances in which a treatment that works well for one of the diseases (e.g., a certain pharmaceutical drug) works for the other disease, too.
To complement the human disease network, the aforementioned U.S. and Korean scientists have also developed what they call a “disease gene network.” Instead of portraying how various diseases are connected by genes, the disease gene network depicts how various genes are connected by diseases. Here, nodes represent genes, while branches represent diseases. The size of each node reflects the number of disorders known to be associated with that particular gene.
Benefits of Gene Association Mapping
Many biologists argue that gene-disease networking is not only a first step toward making GWAS data useful for health care, but also toward completely revamping how physicians think about disease–in particular, how they categorize various illnesses. It is hoped that, instead of viewing human sickness in terms of the tissue involved (e.g., cancer of the breast), physicians will start thinking in terms of the molecular pathways involved. To some extent, doctors are already doing this with diseases like invasive breast cancer, which is categorized as being either Her2/neu-positive or Her2/neu-negative. Her2/neu is a receptor on the surface of breast cancer cells that is coded for by the Her2/neu gene. Women diagnosed as Her2/neu-positive have many more of these receptors and are more likely to benefit from certain forms of treatment than are Her2/neu-negative patients (Burstein, 2005).
Eventually, some scientists say, all diseases will be thought of in terms of which alleles (or genes) a patient has, or at least which alleles are active. As science writer Andrew Pollack described in the aforementioned New York Times article, shifts in the way scientists think about disease have occurred throughout history. For example, consider the work of Carolus Linnaeus, the eighteenth-century Swedish naturalist credited with devising a system of classifying organisms (e.g., into species and genera) that is still used today. Linnaeus also categorized diseases into 11 classes: painful disease, motor disease, and so on. Approximately 100 years later, when the stethoscope was developed, physicians starting thinking about anatomy instead of symptoms and were able to detect problems, such as high blood pressure, in patients with no obvious symptoms. Now, with scientists’ tremendous and rapidly growing understanding of the genetic and molecular basis of human disease, medicine might be on the verge of yet another radical shift–this time, from consideration of anatomy and symptoms to consideration of genes.
As Pollack explained, not everybody is convinced that this shift will be beneficial. Some scientists think that many ambiguities will remain–for instance, there are already cases in which people diagnosed with one or another genetic variant end up never showing the symptoms typically associated with that variant. More serious is the fact that most polygenic diseases, like heart disease and cancer, are caused by more than genetic variations. The environment also plays a vitally important role in human disease–one that cannot be determined by mapping alone. Despite the uncertainties associated with environmental factors, GWAS data and gene-disease networking represent significant steps in the journey toward a fuller understanding of the role of genetics in human disease. As a result of these efforts, associations between seemingly unrelated diseases are coming to light, and researchers are discovering treatment options that might not have otherwise been considered. Although a shift in the traditional diagnosis and treatment of disease by health care professionals is still a long way off, information regarding molecular pathways and genetic correlations is already proving successful in the treatment of some conditions, and patients stand to benefit even further from continued research in the field.
There is also another project – Human Genome Project. The Human Genome Project (HGP) is an international scientific research project with the goal of determining the base pairs that make up human DNA, and of identifying, mapping and sequencing all of the genes of the human genome from both a physical and a functional standpoint.
What did the Human Genome Project discover?
The Human Genome Project identified the full set of human genes, sequenced them all, and identified some of the alleles, particularly those that can cause disease when they get mutated. Genes can be mapped relative to physical features of the chromosome, or relative to other genes.


